lewis dot structure c2h2|C2H2 Lewis structure, Molecular Geometry, Hybridization & Bond : Manila Lewis Structure for C2H2 (Ethyne) Commonly Tested Lewis Structures. We draw Lewis Structures to predict: -the shape of a molecule. -the reactivity of a molecule and how it . Use our Quick Picks tool to get new combinations of randomly generated Powerball lines. Generate up to 10 lines in one click and have the option to include hot or cold numbers, pairs, or trios. More ToolsHow Check NESA Rwanda Exam 2023 Results (P6, S3, S6) Online NESA Rwanda Exam 2023 Results (P6, S3, S6) Online. To check the results online we have given details step by instructions to check Rwanda REB Results. Step 1: Open your Internet Browser (Like Google Chrome) Step 2: Type in address bar .
PH0 · Lewis Structure of C2H2 (With 6 Simple Steps to Draw!)
PH1 · Lewis Structure for C2H2 (Ethyne)
PH2 · How to Draw the Lewis Dot Structure for C2H2: Acetylene (Ethyne)
PH3 · C2H2 Lewis structure, Molecular Geometry, Hybridization & Bond angle
PH4 · C2H2 Lewis structure, Molecular Geometry, Hybridization & Bond
PH5 · C2H2 Lewis structure
PH6 · C2H2 Lewis Structure, Molecular Geometry
PH7 · C2H2 Lewis Structure Tutorial
PH8 · C2H2 Lewis Dot Structure + Geometry
PH9 · C2H2 (Ethyne) Lewis structure
PH10 · C2H2 (Acetylene
The sixth reel introduces a new dimension, enhancing the thrill and potential for significant wins. Triggering free spins with the Book of Ra scatter is like unlocking a sacred chamber, where the potential for hidden treasures becomes palpable. . Play Book of Ra 6 Deluxe if you are not limited by your budget and enjoy massive, less frequent .OMNIPRESENT - Synonyms, related words and examples | Cambridge English Thesaurus
lewis dot structure c2h2*******In the Lewis structure of C2H2 structure there are a total of 10 valence electrons. C2H2 is also called Acetylene (Ethyne). ---- Steps to Write Lewis Structure for compounds like. C2H2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram. Acetylene or C2H2 is the simplest alkyne and a hydrocarbon that is colorless .
Lewis Structure for C2H2 (Ethyne) Commonly Tested Lewis Structures. We draw Lewis Structures to predict: -the shape of a molecule. -the reactivity of a molecule and how it .The Lewis structure for C2H2, also known as ethyne or acetylene, is a diagram that shows the arrangement of valence electrons and the bonding between atoms in a .
chem101csub. 3.82K subscribers. Subscribed. 35. 16K views 10 years ago. If you are asking "help me with Lewis structures" then you came to the right place for free chemistry .By Priyanka. February 16, 2021. C2H2 Lewis structure, Molecular Geometry, Hybridization & Bond angle. C2H2 is a chemical formula for Ethyne, a gaseous alkyne hydrocarbon. It .
Lewis structure of C2H2 (or Acetylene or Ethyne) contains one triple bond between the two Carbon (C) atoms and two single bonds between Carbon . C 2 H 2 Lewis structure. C 2 H 2 (acetylene or ethyne) has two carbon atoms and two hydrogen atoms. In the C 2 H 2 Lewis structure, there is a triple bond between the two carbon atoms, and . A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. . Chemists normally .The Lewis structure for C2H2, also known as ethyne or acetylene, is a diagram that shows the arrangement of valence electrons and the bonding between atoms in a molecule. This structure is essential in understanding the properties and behavior of C2H2 in chemical reactions. C2H2 is a hydrocarbon compound made up of two carbon atoms and two . The total number of valence electrons in the acetylene or ethyne (C2H2) Lewis dot structure is 10. The molecular geometry or shape of C 2 H 2 is identical to its ideal electron pair geometry i.e., .
The C2H2 Lewis structure refers to the arrangement of atoms and electrons in a molecule of ethyne (C2H2) using Lewis dot diagrams. This involves representing each atom using its chemical symbol and drawing dots around it to represent its valence electrons.2. H. 2. (Acetylene | Ethyne) Lewis Structure. C 2 H 2 (acetylene or ethyne) contains two carbon atoms and two hydrogen atoms. There is a triple bond between carbon atoms and hydrogen atoms are joint with carbon atoms though sigma bonds. There are no lone pairs on carbon or hydrogen atoms. In this tutorial, we are going to learn how to draw the .
Hey Guys,In this video we are going to learn about the Lewis structure of C2H2. It is a chemical formula for Ethyne or Acetylene.To understand the Lewis stru. Steps for Writing Lewis Structures. Example 3.4.1 3.4. 1. 1. Determine the total number of valence electrons in the molecule or ion. Each H atom (group 1) has 1 valence electron, and the O atom (group 16) has 6 valence electrons, for a .
5 Steps to Draw the Lewis Structure of C2H2 Step #1: Calculate the total number of valence electrons. Here, the given molecule is CH2. In order to draw the lewis structure of CH2, first of all you have to find the total number of valence electrons present in the CH2 molecule. (Valence electrons are the number of electrons present in the .
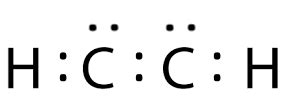
Let's do one more example of a molecule with a multiple covalent bond, so C2H2. So, once again, carbon with four valence electrons, and I have two carbons this time, so I go ahead . Let's do one more example of a molecule with a multiple covalent bond, so C2H2. So, once again, carbon with four valence electrons, and I have two carbons this time, so I go ahead .C2H2 Lewis structure, Molecular Geometry, Hybridization & Bond We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of .
Draw the Lewis structure for C2H2 and provide the following information. (Consider C to be the central atom.) a. total number of valence electrons from all atoms b. number of shared electron pairs around the central atom c. number of unshared electron pai; Draw and explain the electron dot structure for C2H2. Give the Lewis dot structure for ICl2-lewis dot structure c2h2 C2H2 Lewis structure, Molecular Geometry, Hybridization & Bond The Lewis structure of C 2 H 2 is a highly unreactive and stable compound with one single bond and one triple bond in it, as well as fulfilling all the rules of octave rules, making it more stable. The Lewis structure also known as the dot structure of an acetylene or ethyne molecule is mainly given to represent the arrangement of valence .
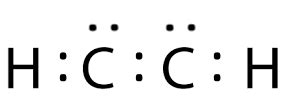
Hence, the electron dot structure of ethyne C 2 H 2 has been drawn above. Suggest Corrections. 35. Q. Draw the electron dot structure of ethyne and also draw its. structural formula. Q. Question 30. Drawthe electron dot structure of ethyne and also draw its structural formula. Q. Draw electron dot structure of ethyne. Q.
Step 3: Connect each atoms by putting an electron pair between them. Now in the C2H2 molecule, you have to put the electron pairs between the carbon-carbon atoms and between the carbon-hydrogen atoms. This indicates that these atoms are chemically bonded with each other in a C2H2 molecule. Step 4: Make the outer atoms stable. A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and .Bonding in Ethane. In the ethane molecule, the bonding picture according to valence orbital theory is very similar to that of methane. Both carbons are sp 3-hybridized, meaning that both have four bonds arranged with tetrahedral geometry.The carbon-carbon bond, with a bond length of 1.54 Å, is formed by overlap of one sp 3 orbital from each of the carbons, .This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Question: Draw the Lewis structure for acetylene (C2H2). Be certain you include any lone pairs. Draw the Lewis structure for acetylene (C2H2). Be certain you include any lone pairs.PROBLEM 4.2.4 4.2. 4. Methanol, H 3 COH, is used as the fuel in some race cars. Ethanol, C 2 H 5 OH, is used extensively as motor fuel in Brazil. Both methanol and ethanol produce CO 2 and H 2 O when they burn. Write the chemical equations for these combustion reactions using Lewis structures instead of chemical formulas.
Poki; 2 Player Games; Tank Trouble 2; Tank Trouble 2. Subterranean Software 4.3 175,525 votes. Tank Trouble 2 is an online war game where you blast bouncing missiles at enemy war vehicles. Tank Trouble 2 pits you against clever army generals in mazelike battlefields. In Solo mode, you will face Laika, a master of war.
lewis dot structure c2h2|C2H2 Lewis structure, Molecular Geometry, Hybridization & Bond